Overcome the challenges of PMS2 variant calling with Devyser LynchFAP – our new NGS solution
Devyser LynchFAP is the latest addition to Devyser’s expanding hereditary cancer screening...
Please find the terms and conditions for different countries and regions below.
| Country | Legal terms |
|---|---|
| Austria | Terms and conditions |
| Benelux | Terms and conditions |
| Germany | Terms and conditions |
| Switzerland | Terms and conditions |
| United Kingdom | Terms and conditions |
| Nordics | Terms and conditions |
| North America | Terms and conditions |
| Distributor sales | Terms and conditions |
| France | Terms and conditions |
News | June 26, 2025
Devyser launches Devyser HLA Loss: A high-precision NGS assay for post-transplant researchNews | October 29, 2024
Devyser seeks to secure FDA approval for NGS test for kidney transplant monitoringNews | October 28, 2024
Devyser Compact achieves Class III approval in China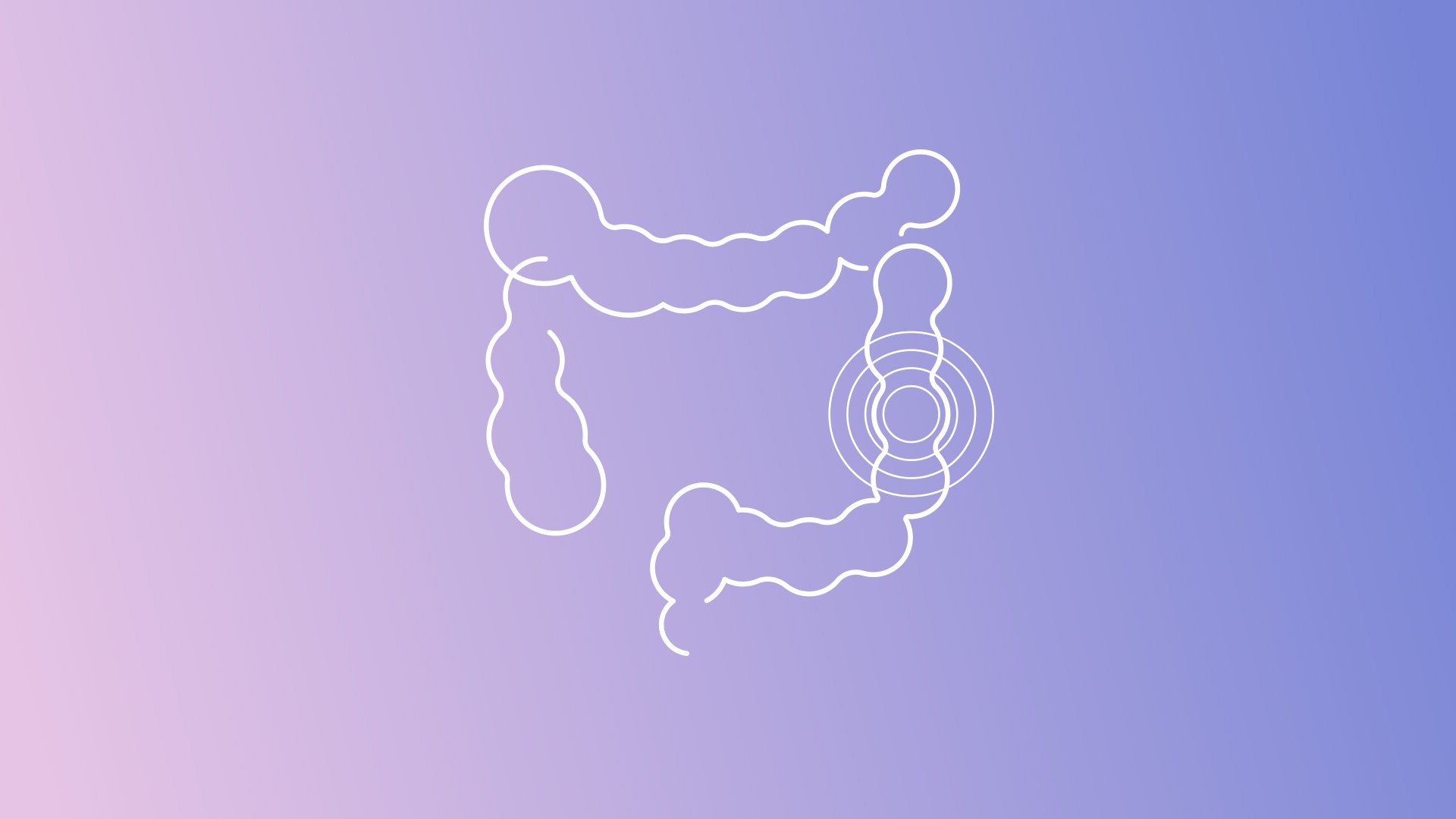
Devyser LynchFAP is the latest addition to Devyser’s expanding hereditary cancer screening...
Read More
-2.png)
Devyser is thrilled to announce the launch of two new products, Devyser LynchFAP and Devyser BRCA...
Read More

As a lab manager, you know the importance of streamlining workflows to maximize productivity and...
Read More

Do you know a man who has participated at least once in “Movember”? Every year, thousands of men...
Read More