Why choose Advyser Solid organs?
Targeted design
From lab technicians seeking efficiency to nephrologists making critical patient care decisions, Advyser Solid organs supports a complete set ecosystem within transplant monitoring.
Advanced analytics
Utilizing a panel of genetic markers Advyser Solid organs offers unmatched quantification accuracy, setting new standards in the management of post-transplant patients.
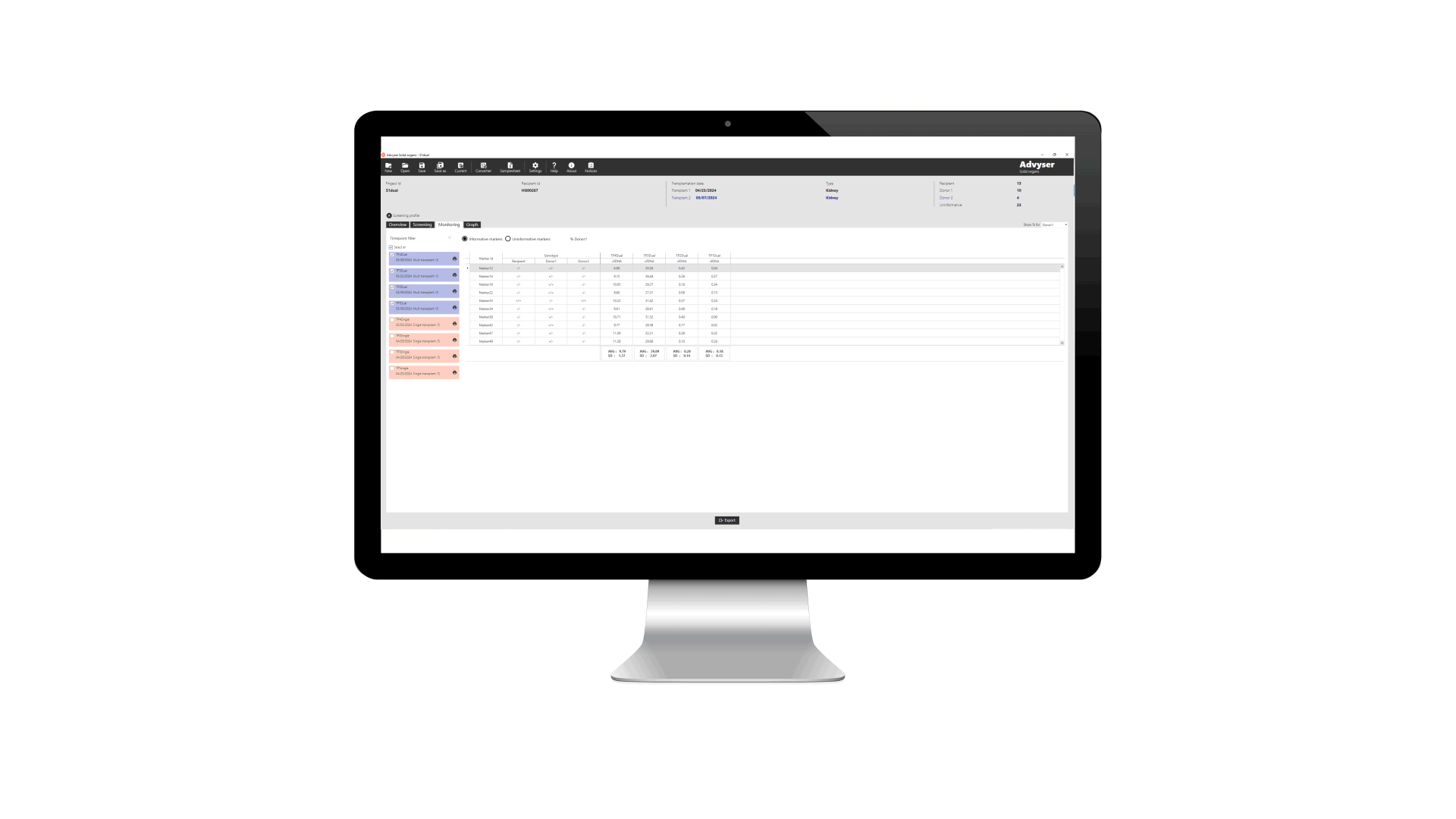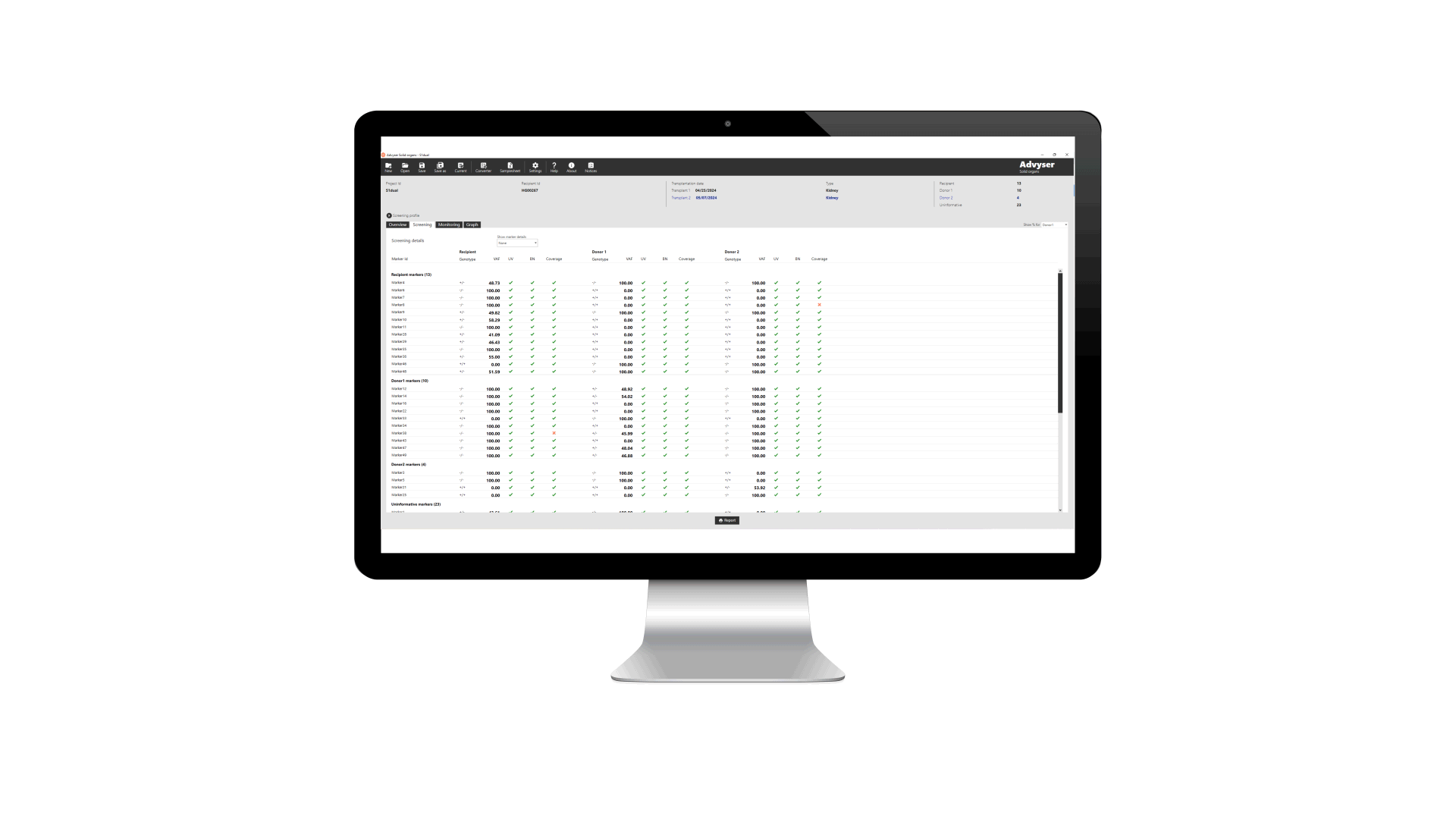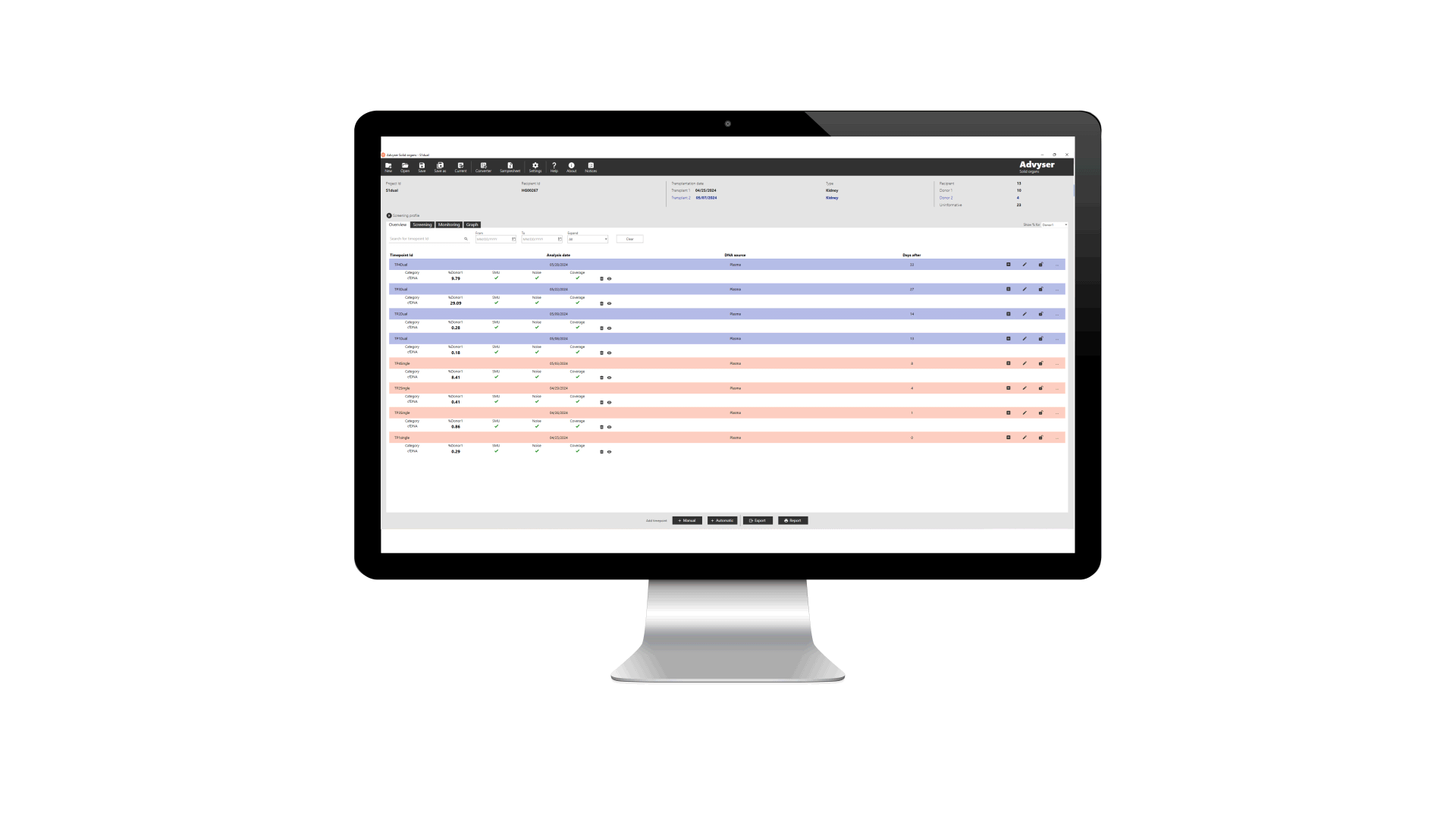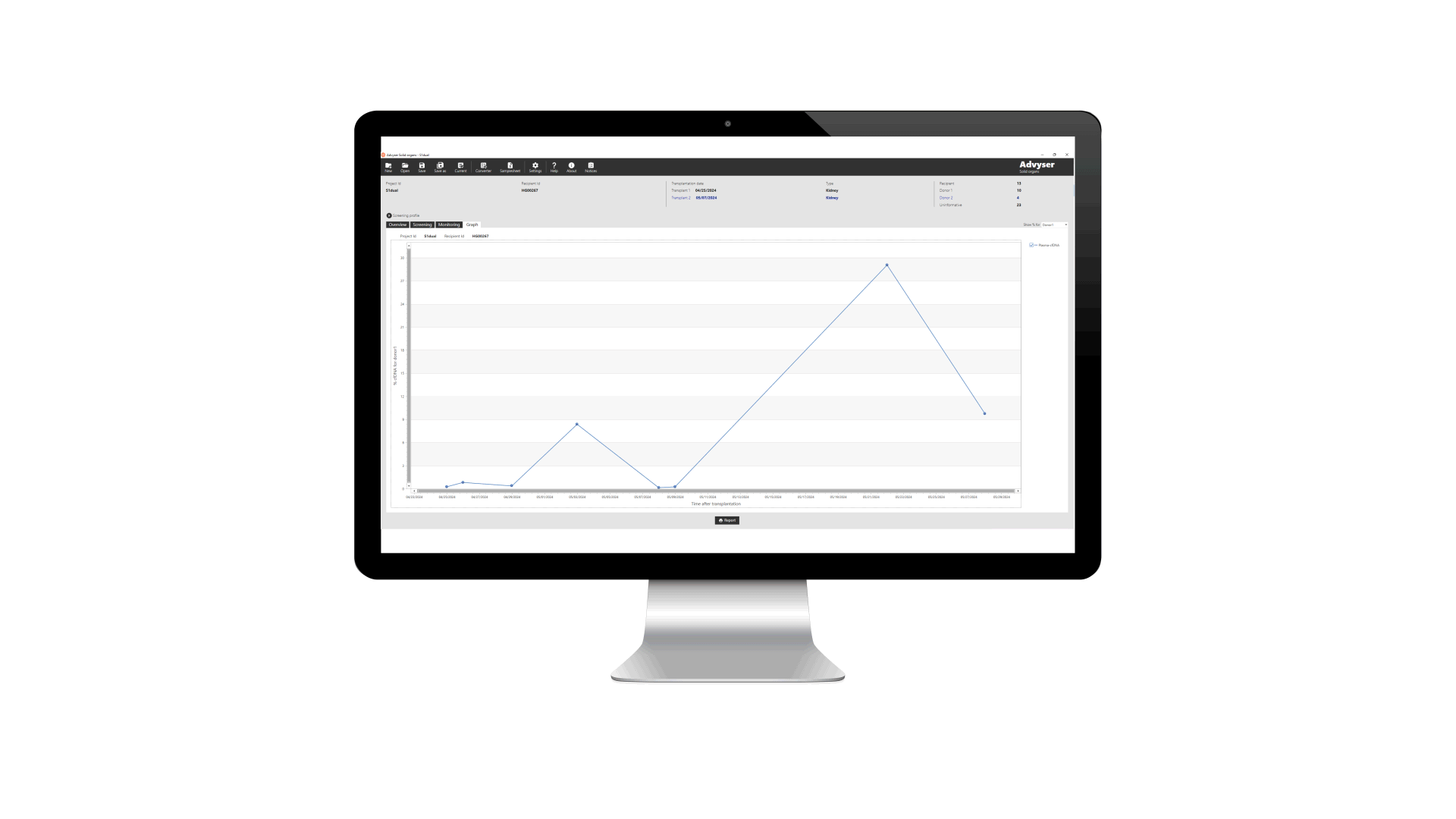
Key features of Advyser Solid organs
Advyser Solid organs is an IVDR-certified software solution for monitoring donor-derived cell-free DNA (dd-cfDNA) in patients following kidney transplantation. The software is intended for use with One Lambda Devyser Accept cfDNA, a novel NGS test for detecting dd-cfDNA in blood samples from kidney transplant patients.

IVDR certified
Devyser is striving to meet customers demand of compliance and quality in the products we provide. This certainly includes the in-house designed software Advyser Solid organs.

Easy workflow
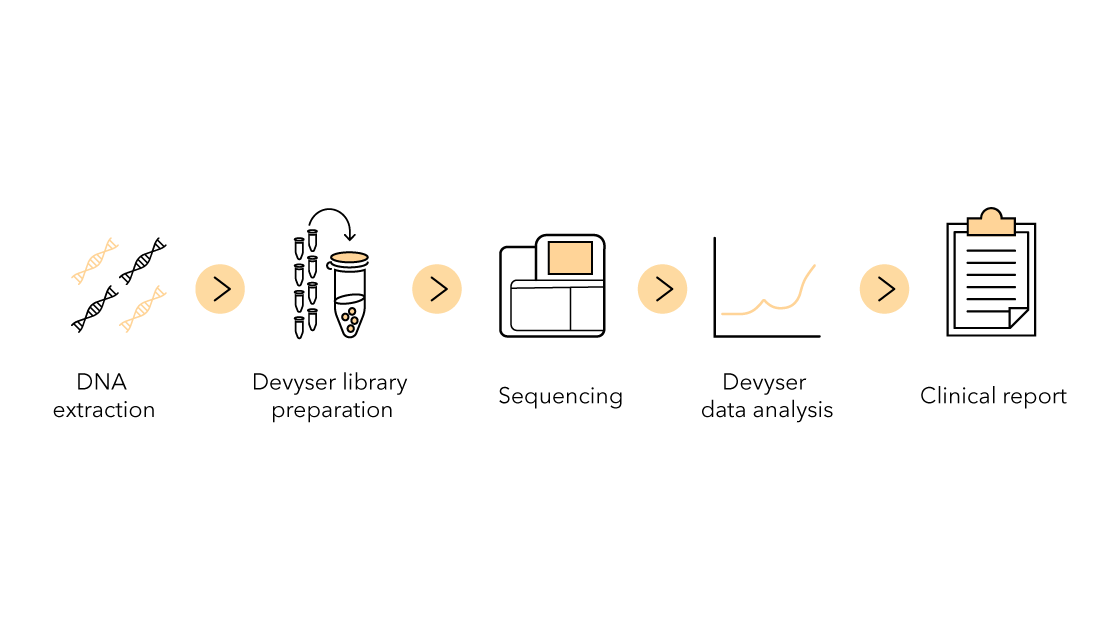
Advyser Solid organs is an in-house developed software, to fit perfectly with the assay OneLambda Devyser Accept cfDNA, which is why users will benefit from a more streamlined and consistent software experience.

Actionable results
Advyser software solution is highly robust and tailored to the specific requests of current users. The software specializes in the swift and accurate analysis and reporting of assay results. This customized software application has received exceptional user ratings, reflecting the Devyser commitment to meet precise user requirements.
An end-to-end solution
Advyser Solid organs is a software solution for post-transplant monitoring, a tailored software for the One Lambda Devyser Accept cfDNA NGS-based assay. It is an end-to-end and highly robust solution to meet the specific requests of users. The software is IVDR-certified and hence meets the established safety, efficacy, and quality requirements.
Contact us
Want to know more about Advyser Solid organs or other post-transplant monitoring products?
Complete the form below and we will be in touch promptly.

Analysis made easy
Fast and effective test data analysis is crucial to achieving accurate outcomes and efficient workflows.
Dedicated software makes analyzing test results quick, easy and trouble-free. For lab personnel, this means a streamlined, end-to-end process with unmatched reliability and transparency.
