Devyser announces study publication demonstrating dd-cfDNA detection in dual donor kidney transplant patients
Devyser Diagnostics AB announces today an article titled “Detection of donor-derived cell-free DNA...
Eliminate cell culture, increase analysis success rates and speed up your pregnancy loss aneuploidy analysis.
8
Rapid aneuploidy analysis of chromosomes 13, 15, 16, 18, 21, 22, X and Y
5
Results in less than 5 hours
No tissue culture required

Women who have undergone one or more spontaneous abortions caused by chromosomal abnormalities are at increased risk for chromosomal abnormalities in future pregnancies. Cytogenetic studies of miscarriages are highly recommended even in the case of the first pregnancy loss. Identification of the possible cause of fetal loss significantly reduces the long-term psychological distress associated with a miscarriage and enables improved genetic counseling in future pregnancies. The most frequently observed numerical chromosomal abnormalities involve chromosomes 13, 15, 16, 18, 21, 22 and X.
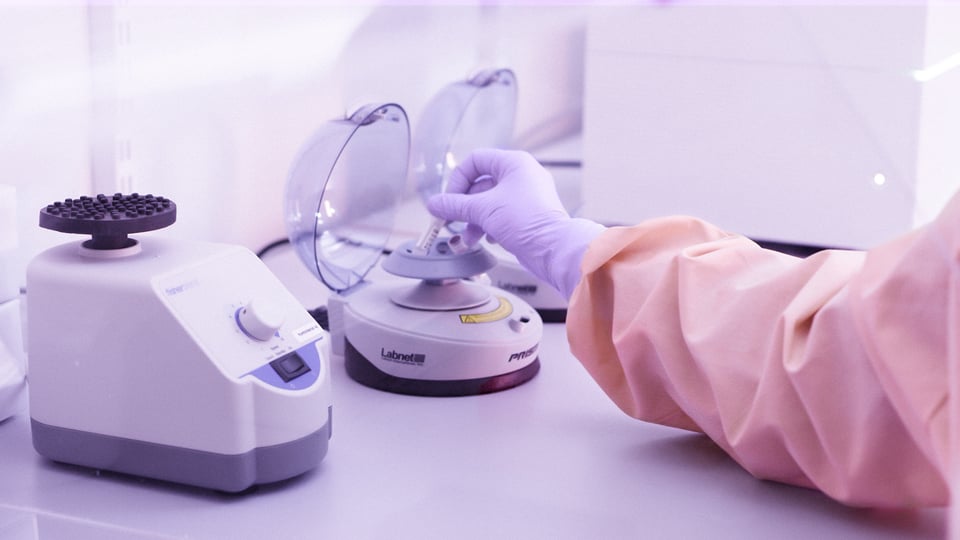
Conventional cytogenetic studies (karyotyping or FISH) are expensive and need a long period of time in order to obtain results. Moreover, they result in high rates of culture failure, misdiagnosis due to maternal contamination and cell overgrowth as well as insufficient quality of chromosome preparations. Cell culture may selectively yield normal karyotypes or selected abnormal karyotypes that survive in-vitro cell proliferation.

QF-PCR does not require cell culture, requires minute amounts of tissue material (Products of conception - POC) and allows the lab to obtain results within one working day. The Devyser Extend kit includes 42 genetic markers for aneuploidy analysis by QF-PCR of chromosomes 13, 15, 16, 18, 21, 22, X and Y. Two unique X-chromosome counting markers for reliable detection of Turner syndrome are also included in Devyser Extend.
Instructions for use
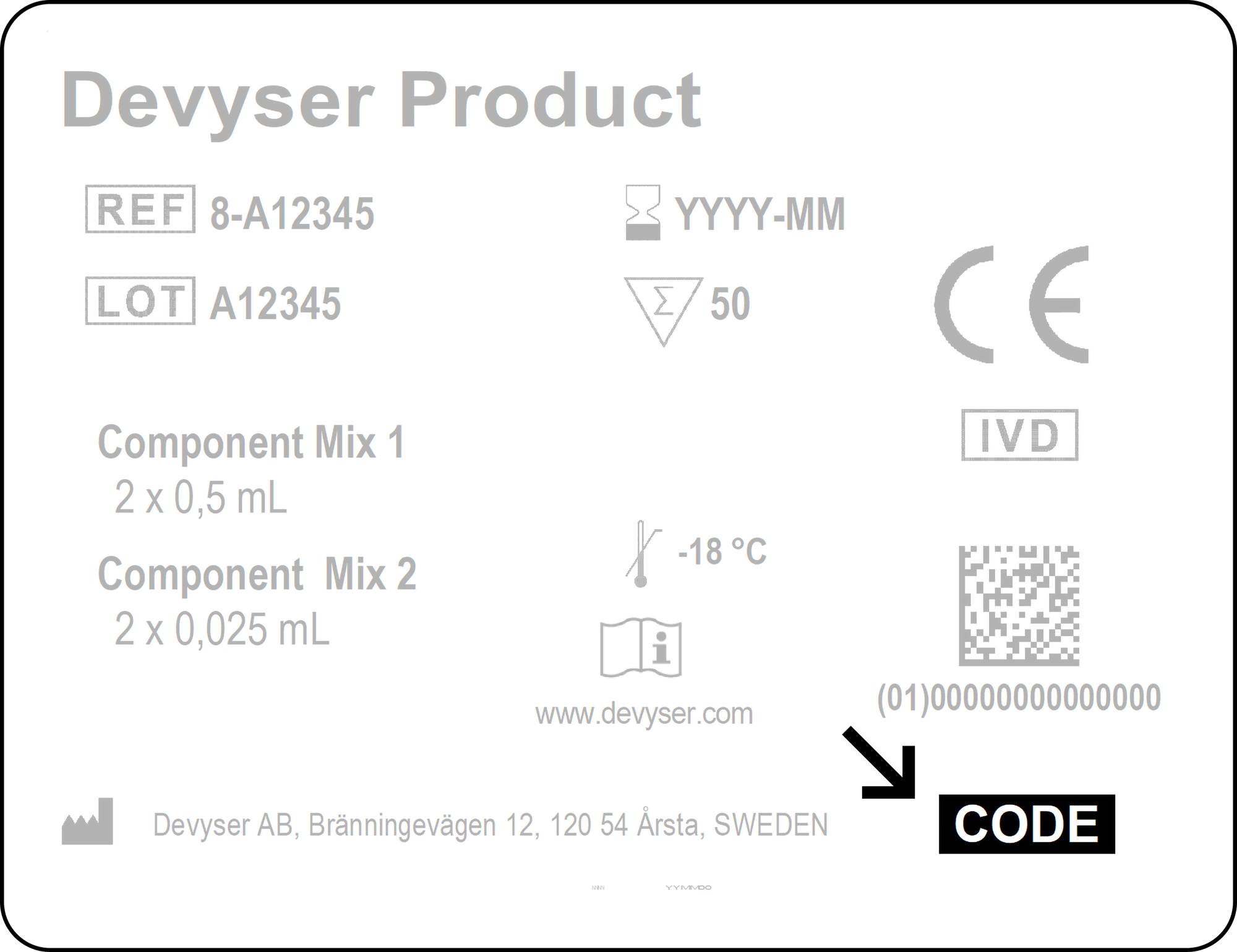
Enter access code found in the lower right corner of the label on the kit box.
Certificates
Download a specific Batch Release Certificate (BRC) below.
Guidelines and handbooks (6)
Addendum to handbook Devyser SeqStudio QF-PCR products 7-B910 v2023-03-30 (English)
490 kb pdf
Addendum to handbook Devyser VeritiPro QF-PCR products 7-B904 v2023-03-31 (English)
100 kb pdf
Product information (3)
Software settings (8)
Safety data sheets (1)
Data tables (0)
Enter access code found in the lower right corner of the label on the kit box.

Download a specific Batch Release Certificate (BRC) below.
Addendum to handbook Devyser SeqStudio QF-PCR products 7-B910 v2023-03-30 (English)
490 kb pdf
Addendum to handbook Devyser VeritiPro QF-PCR products 7-B904 v2023-03-31 (English)
100 kb pdf
CE-IVD
Devyser Extend M1 v2
Aneuploidy determination of chromosomes 15, 16 and 22
8-A015.2-M1
Pack size: 25
CE-IVD
Devyser Extend v2
Aneuploidy determination of chromosomes 13, 15, 16, 18, 21, 22, X and Y
8-A015.2
8-A015.2-100
Pack size: 25
Pack size: -100
RUO
Devyser Extend M1 v2
Aneuploidy determination of chromosomes 15, 16 and 22
8-A015.2M1RUO
Pack size: 25
RUO
Devyser Extend v2
Aneuploidy determination of chromosomes 13, 15, 16, 18, 21, 22, X and Y
8-A015.2-RUO
Pack size: 25
Complete the form below and we will be in touch promptly.

.png)
Devyser Diagnostics AB announces today an article titled “Detection of donor-derived cell-free DNA...
Read More

Read More

Devyser’s novel test for detecting donor-derived cell-free DNA in blood samples from...
Read More
.jpg)
A better patient care is the final objective for Dr. Helena Devos and the team at Sint Jan’s...
Read More
Fast and effective test data analysis is crucial to achieving accurate outcomes and efficient workflows.
Dedicated software in our products makes analyzing test results quick, easy and trouble-free. For users, this means a streamlined end-to-end process with unmatched reliability and transparency.
"
Disclaimer: Products mentioned here are CE-IVD marked but not FDA-cleared. Availability in each country depends on local regulatory marketing authorization status. Please consult your local sales representative for details.