.png)
News | May 30, 2024
Devyser wins tender for oncology NGS solutions in Italy
Find out more

.png)



Devyser´s annual report 2023 has today been published on the company´s website. The report is...
-2.png)
Devyser has secured a CFTR NGS proposal with UNC Hospitals. The proposal with UNC Hospitals is...
.png)
Devyser Diagnostics AB announces today an article titled “Detection of donor-derived cell-free DNA...
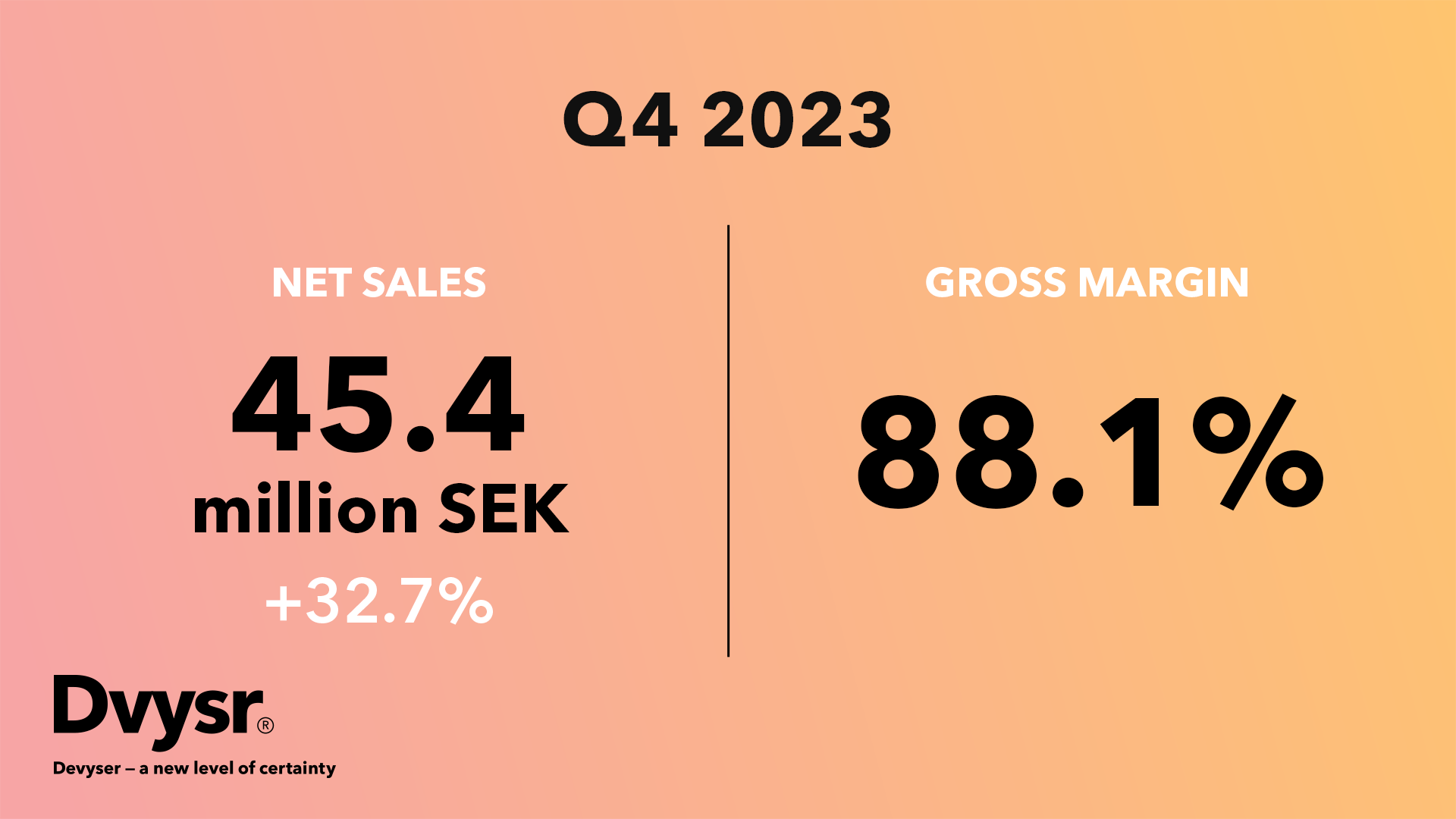
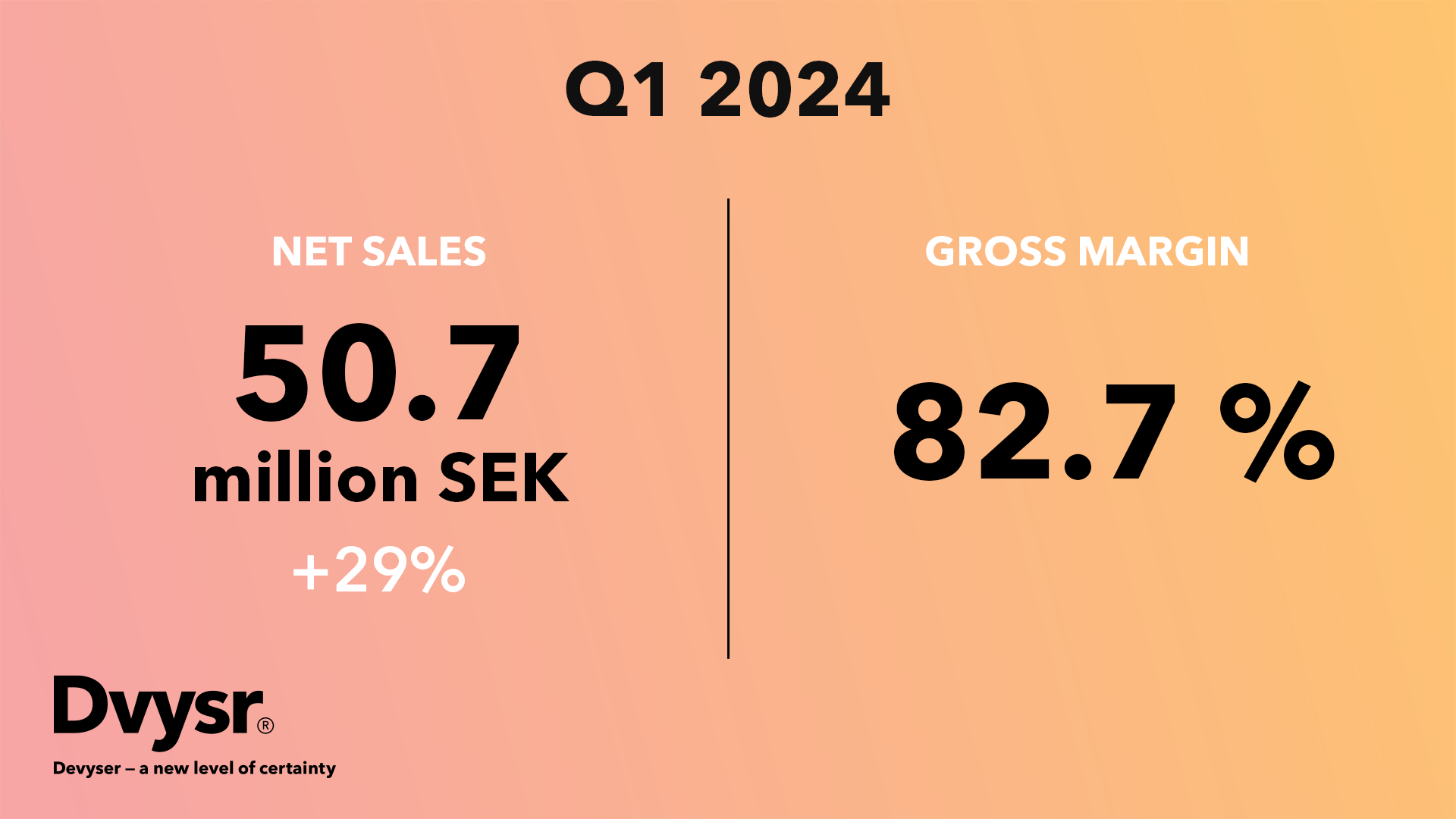
“Devyser is able to report strong sales growth and record high gross margins for the fourth...


Devyser Genomic Laboratories, Devyser's US-based CLIA-certified laboratory, has signed its first...

Devyser has been awarded a tender for Devyser’s CFTR fragment analysis products, that are used for...